
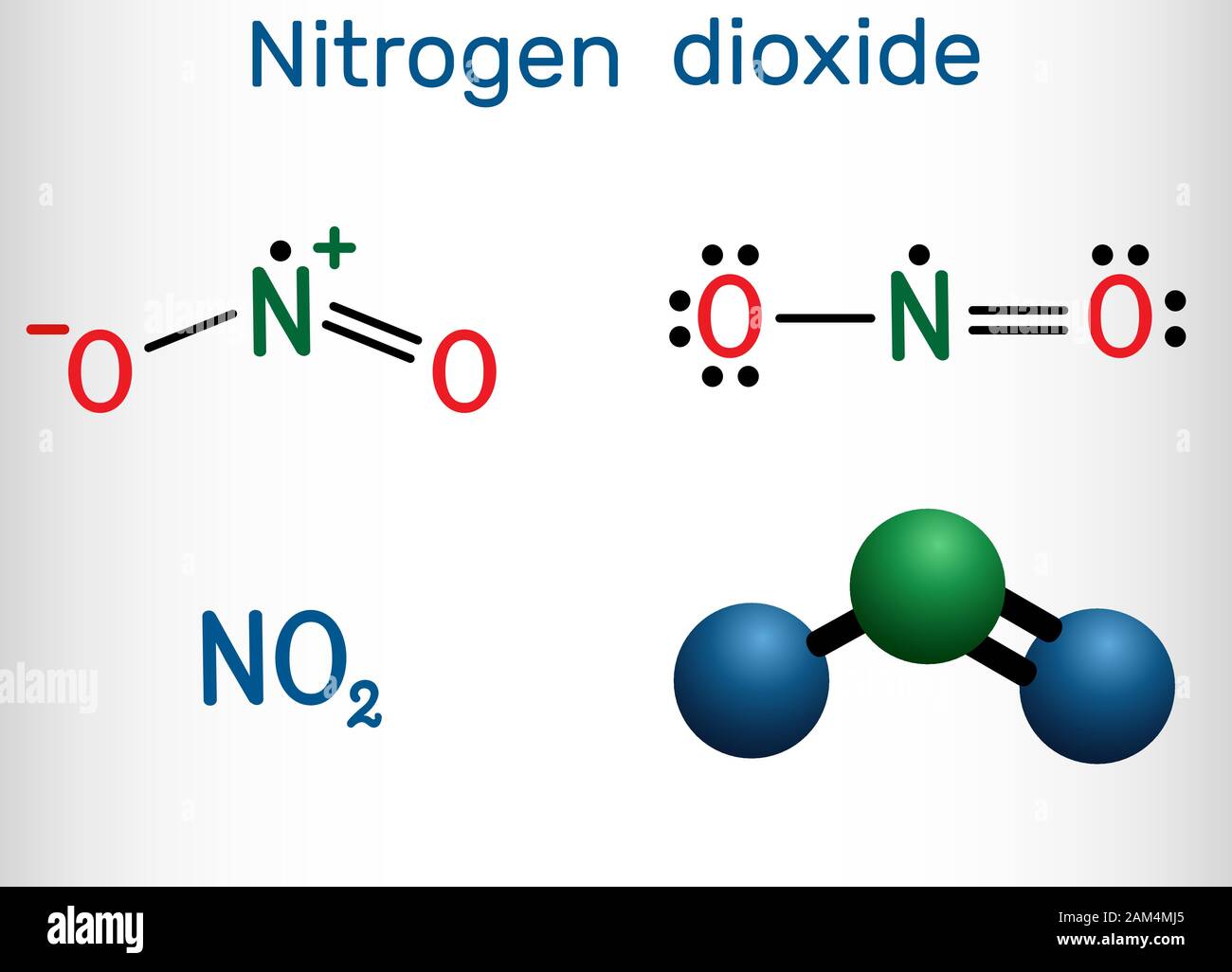
NO2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
Geometry. NO2- Geometry and Hybridization. Nitrogen is the central atom: There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent bonds. Both oxygens get 6 electrons as three lone pairs, and nitrogen gets one lone pair: One lone pair from an oxygen is used to make a π bond with the nitrogen and thus making the ionic.
N2 Structure
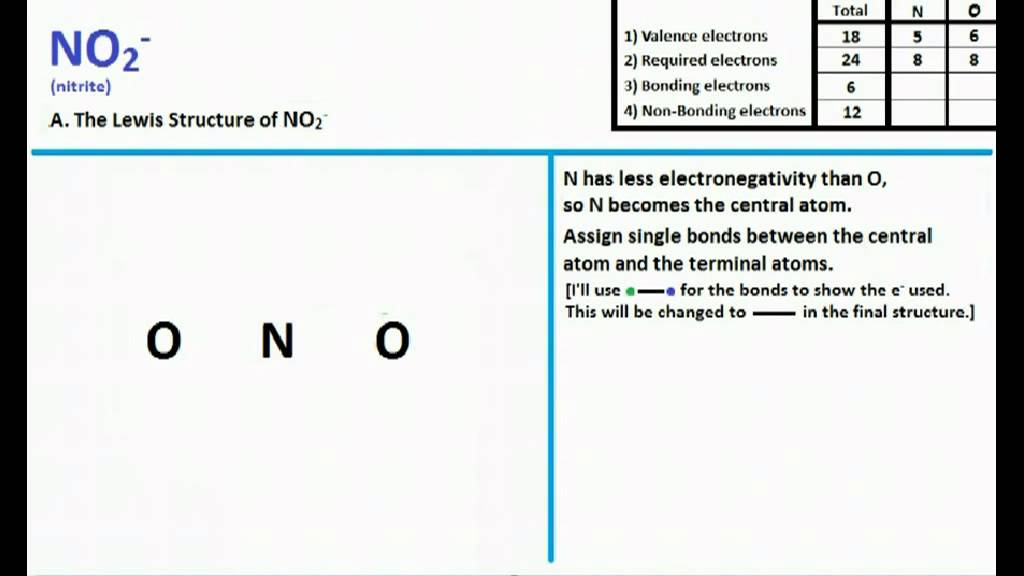
For the NO2- Lewis structure, calculate the total number of valence electrons for the NO2- molecule. After determining how many valence electrons there are in NO2-, place them around the central atom to complete the octets. There are a total of 18 valence electrons for the Lewis structure for NO2-. Nitrogen is the least electronegative atom in.

NO2 Molecular Geometry / Shape and Bond Angles YouTube
The Nitrogen atom in the Lewis structure for NO 2 is the least electronegative atom and passes at the center of the structure. Molecular Geometry and Bond Angles of NO 2. Since the Nitrogen Dioxide (NO 2) has an extra electron in a nitrogen atom orbital, it will result in a higher degree of repulsions. However, if we consider one lone electron.

How to draw NO2+ Lewis Structure? Science Education and Tutorials
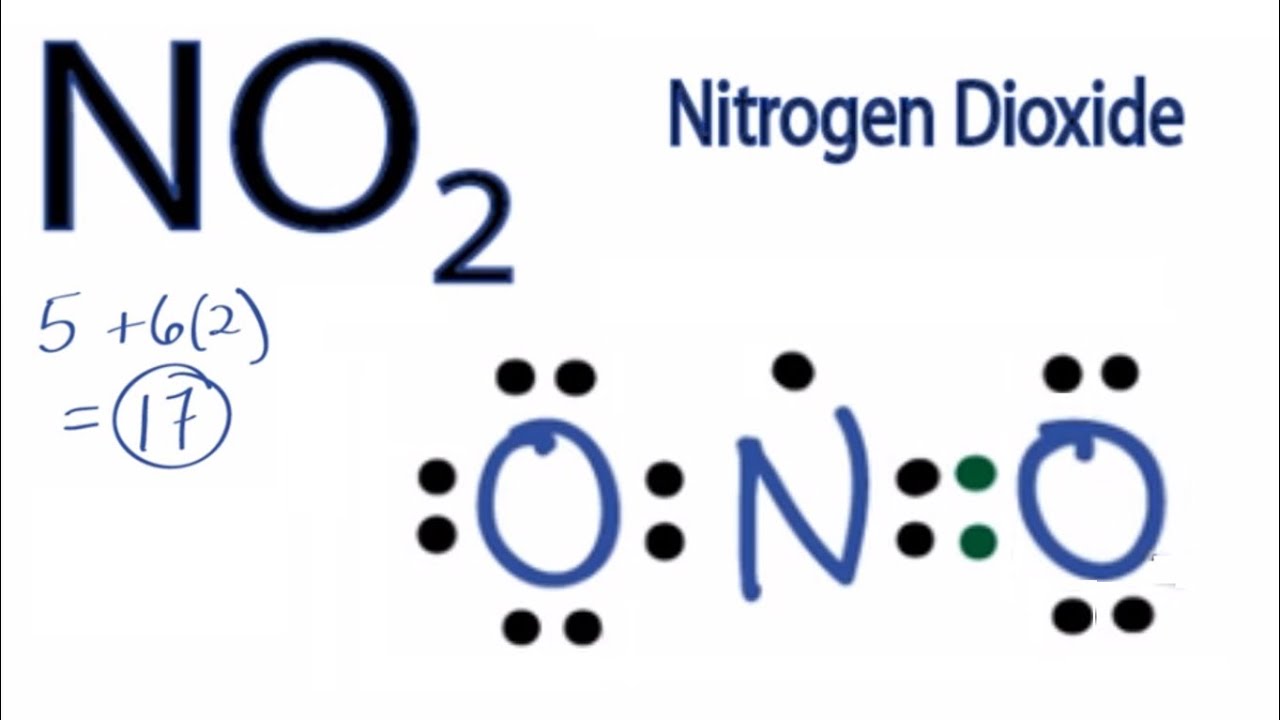
A step-by-step explanation of how to draw the NO2 Lewis Structure (Nitrogen Dioxide). The NO2 Lewis structure has a total of 17 valence electrons. It's n.
NO2 Lewis Structure and Molecular Geometry YouTube
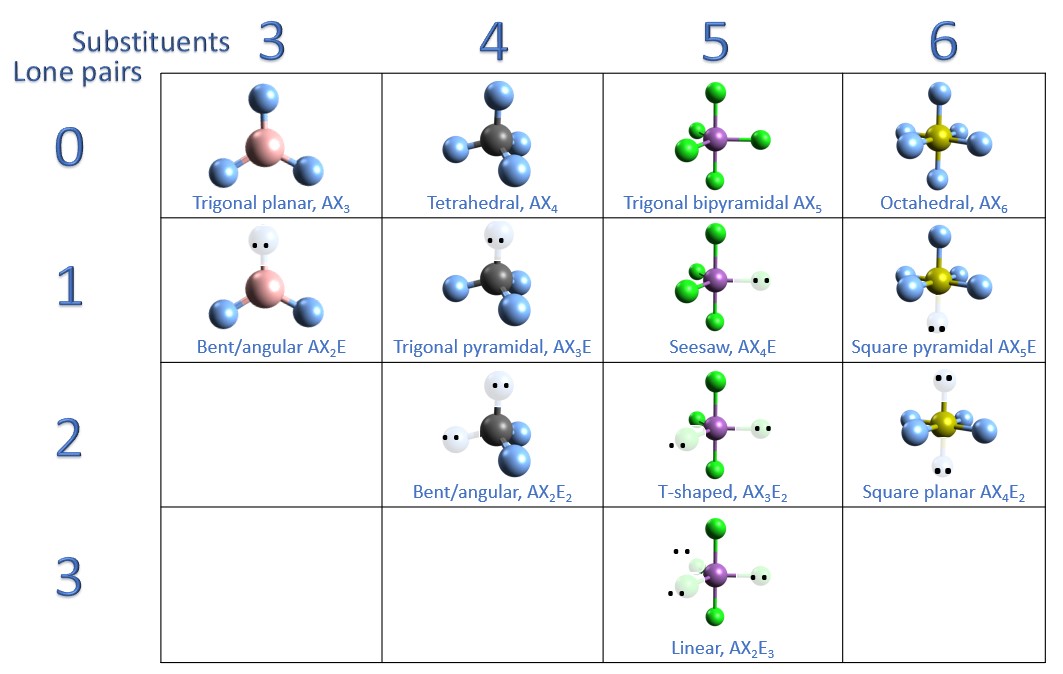
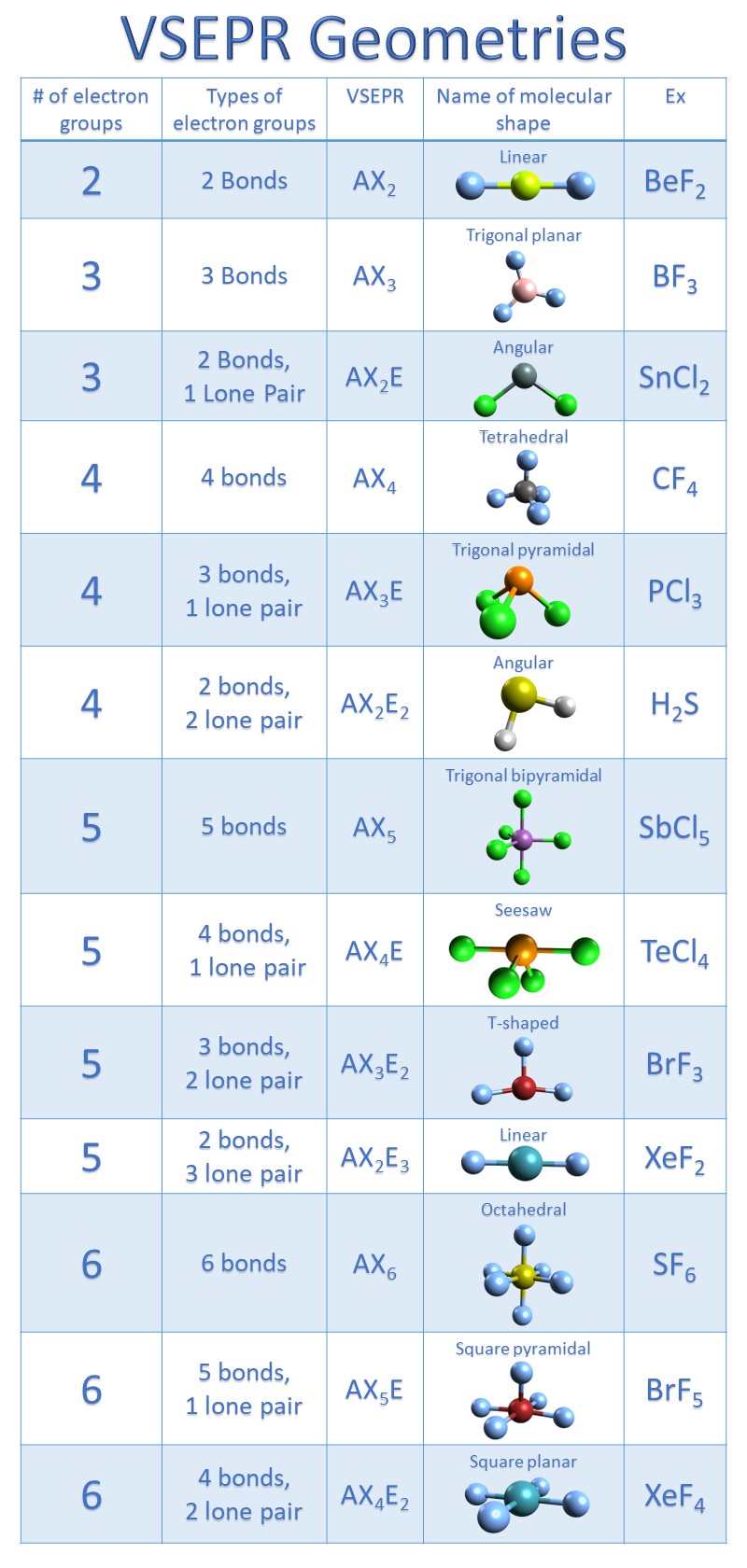
Figure 8.6.1 8.6. 1 shows the various molecular geometries for the five VESPR electronic geometries with 2 to 6 electron domains. When there are no lone pairs the molecular geometry is the electron (VESPR) geometry. When there are lone pairs, you need to look at the structure and recognize the names and bond angles.

Schematic molecular orbital diagram for NO2, NO 2 δ + , and NO 2
Electron Geometry of NO2. The electron geometry of a molecule is determined by the arrangement of electron pairs around the central atom. In the case of NO2, nitrogen is the central atom, and it has one lone pair and two bonding pairs. According to the VSEPR model, the presence of one lone pair and two bonding pairs gives NO2 an electron pair.

Overview NO2+ electron and molecular geometry
There is an easy three-step process for determining the geometry of molecules with one central atom. Step 1: Determine the Lewis structure of the molecule. For NO 2-, it is as shown below: For a full-explanation of how to figure out the Lewis structure, please go to Lewis Structure of NO 2-. Step 2: Apply the VSEPR notation to the molecule.

Lewis structure of NO2. How to draw the Lewis structure of NO2. Advance
A quick explanation of the molecular geometry of NO2 including a description of the NO2 bond angles. Note the exact bond angle is 134.3 degrees).Looking at.

13+ Lewis Structure Of No2 Robhosking Diagram
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer. Question: What is the Lewis structure for NO2- ? Then, what is its electron geometry, hybridization, molecular geometry, and polarity? What is the Lewis structure for NO2- ? Then, what is its electron.

How to draw NO2+ Lewis Structure? Science Education and Tutorials
VSEPR chart: We can see that NO2 has a bent molecular geometry and the angle is around 120 degrees. But here we have some exceptions. In NO2, we have 2 Bond Pairs and 1 lone electron. If we look at the nitrite ion NO2-, we have 2 Bond Pairs and 1 Lone pair of electrons.
Molecular Geometry of NO2 [with video and free study guide]
This chemistry video tutorial explains how to draw the lewis structure of NO2-, the Nitrite ion.Chemistry - Basic Introduction: https://ww.
Molecular Geometry of NO2 [with video and free study guide]
The total valence electrons available for drawing nitrite [NO2]- ion Lewis structure are 18. The molecular geometry or shape of NO 2- is bent or V-shaped. The ideal electron geometry of NO 2- is trigonal planar. The central N-atom has sp 2 hybridization in NO 2-. The O=N-O bonded atoms form a mutual bond angle of 134°.

NO2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
A quick explanation of the molecular geometry of NO2 - (the Nitrite ion) including a description of the NO2 - bond angles.Looking at the NO2 - Lewis structur.

How do you draw the lewis structure for NO2?
Total energies, population analyses, and one‐electron orbital energies vs angle (Walsh diagrams) have been computed to aid interpretation of the wavefunctions. It turns out that the dramatic change in geometry from NO 2 + (linear) to NO 2 − (bent, 115.4°) can be understood by the occupancy and shape of a single orbital, the 6a 1 in NO 2 − .

NO2F Lewis Structure, Molecular Geometry, Hybridization, and Polarity
One can infer the hybridization of nitrogen in NO2 from the molecular shape of the molecule. The bent or V-shaped molecular geometry of NO2 is consistent with sp2 hybridization, as it allows for the repulsion between the lone pair of electrons and the bond pairs. V. Electron Geometry of NO2 A. Determination of electron geometry of NO2
Nitrogen Dioxide No2 Molecule Structural Chemical Free Nude Porn Photos
A step-by-step explanation of how to draw the NO2 - Lewis Dot Structure (Nitrite ion).For the NO2 - structure use the periodic table to find the total number.